ElectriStor™ — How It Works
In a typical battery that most people are familiar with, both the power-producing and energy-storage elements are contained in a single physical package. In a redox flow battery, the power and energy storage elements are physically separate and decoupled. This construction provides for a maximum degree of system design flexibility and scalability not available in conventional batteries. The electroactive materials that store electrical energy are dissolved in a supporting liquid and the complete solution is called an electrolyte. The electrolytes are contained in tanks external to the means of energy conversion, and the amount of energy stored is proportional to the amount of electrolyte contained in the tanks.
Basic Redox Flow Battery System Operation
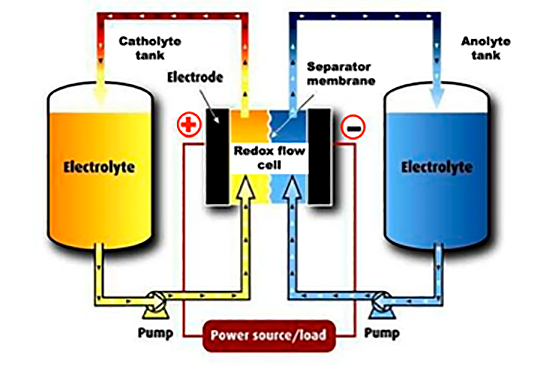
Electrical power is produced in an electrochemical device called a redox flow cell as shown in the figure above. This flow cell can either produce electrical energy or absorb it, as electrons are transferred from current collectors to or from the dissolved active materials in the electrolytes. Arrays of these cells are built into a stack to produce a more usable quantity of power. This stacked array of cells is also referred to as an energy converter stack since it is converting electrical energy to electrochemical energy and vice versa. More power requires a larger energy converter; more energy requires more electrolyte. This power and energy independence, provided only by a flow battery system, allows electrical engineers to cost-effectively size both the power and energy capabilities of any system to virtually any stationary energy-storage application. This means flow battery systems can service a much broader range of applications than any other stationary energy storage solution, even lithium-ion based systems.
To start a typical charge/discharge cycle let’s begin by charging the system. To do this, we pump discharged electrolytes through the energy converter while external electrical power is applied in a controlled manner, and the electrolytes charge, thereby storing electrical energy. We stop pumping when we sense the system is fully charged or when there is no electricity available for storage.
The next part of the cycle is discharge. To do this, we now pump the charged electrolytes from the external tanks through the energy converter, electrons from the negative electrode flow through an external circuit to the positive electrode, and electrical power is now generated to power an external load. Thus, during discharge the electrical energy stored in the electrolyte is depleted as it does useful work. We stop pumping when we sense the system is fully discharged or until there is no further demand for the stored electricity.
The entire charge/discharge process is fully reversible and either partial or full charge/discharge cycles are possible. Most important, there are no chemical or physical changes in the energy converter that can create durability problems as they do in conventional batteries. There is only a change in the chemical state of the electrolytes; and the energy converter itself is not altered in any way, with the entire system ready for the next charge/discharge cycle.
